- Home
- About Us
- Injectable Products
- Ampicillin and Sulbactam for Injection, USP
- Ampicillin for Injection, USP
- Calcium Gluconate in Sodium Chloride Injection
- Cefazolin for Injection, USP
- Cefepime for Injection, USP
- Cefoxitin for Injection, USP
- Ceftazidime for Injection, USP
- Ceftriaxone for Injection, USP
- CISplatin Injection
- CISplatin for Injection, USP
- Dexmedetomidine HCl Injection
- Dexmedetomidine HCl in 5% Dextrose Injection
- Esmolol Hydrochloride in Sodium Chloride Injection
- Esmolol HCI in Water for Injection
- Ertapenem for Injection
- Imipenem and Cilastatin for Injection, USP
- Levetiracetam in Sodium Chloride Injection
- Levofloxacin Injection in 5% Dextrose
- Magnesium Sulfate in 5% Dextrose Injection, USP
- Meropenem for Injection, USP
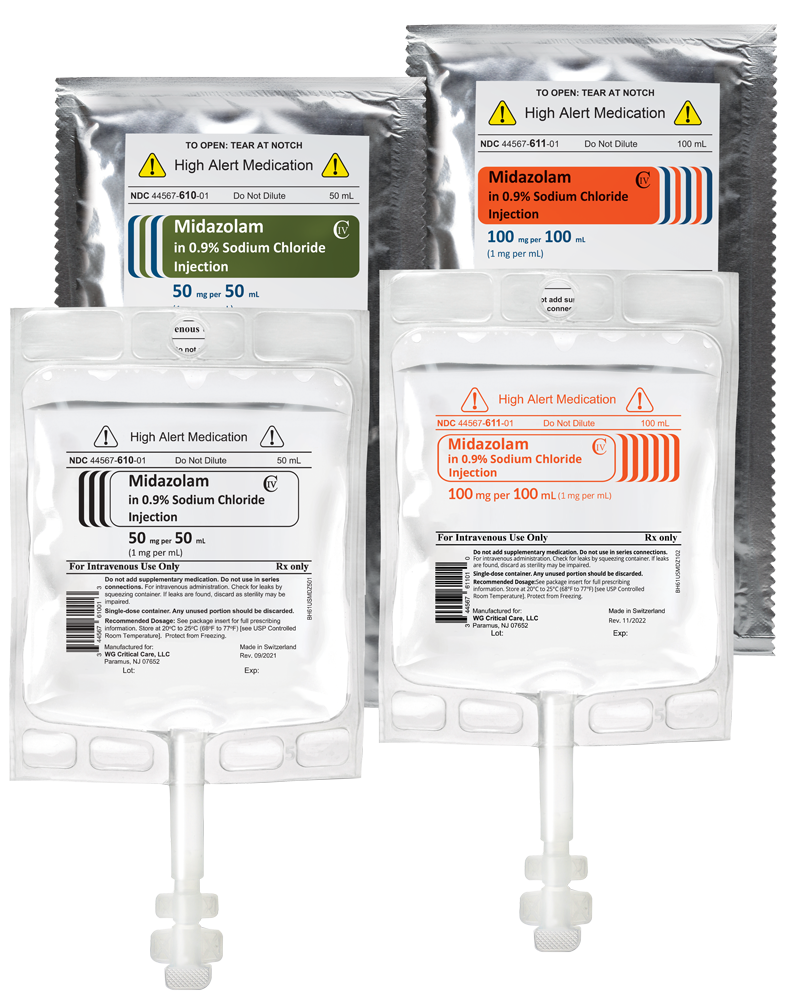
- Midazolam in 0.9 % Sodium Chloride Injection
- Nafcillin for Injection, USP
- Paclitaxel Injection, USP
- Penicillin G Potassium for Injection, USP
- Piperacillin and Tazobactam for Injection, USP
- News
- Contact Us
- See Privacy Policy